- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
Article
AD treatments exhibit long-term relief for other inflammatory skin conditions
The efficacy and remarkable safety profile of two drugs, tacrolimus (Protopic?, Fujisawa Healthcare) and pimecrolimus (Elidel?, Novartis), have revolutionized the treatment of atopic dermatitis (AD) and appear to have promise for a variety of other inflammatory skin conditions that require long-term therapy, according to Alan Fleischer, M.D., professor and chair of dermatology at Wake Forest University School of Medicine.

Topical corticosteroids have historically been the mainstay of AD treatment, but are fraught with safety concerns in continuous use, with potential adverse events such as skin atrophy, hypopigmentation and steroid acne. As a result, the intensity and duration of topical corticosteroid use is seriously restricted.
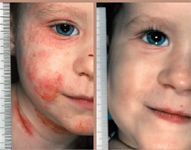
The safety of tacrolimus and pimecrolimus may be attributed in large part to their specificity of action. The drugs are both calcineurin inhibitors and act directly on T-lymphocytes by binding to FK-binding protein, forming a complex that, in turn, binds to calcineurin, a phosphatase that is active only when bound to calcium and calmodulin. This inhibition of calcineurin prevents immuno-dysregulatory responses that are theorized to play a role in AD pathogenesis.
Because the use of topical corticosteroids must be carefully monitored, the safety profile of calcineurin inhibitors may ultimately result in a change in the treatment paradigm, according to Dr. Fleischer.
"The need for monitoring with these drugs is drastically decreased," he says. "I have no hesitancy for patients to use these agents at home in treatment for very early signs of disease."
Use of tacrolimus and pimecrolimus are not, however, without considerations. These drugs may initially be irritating on highly inflamed skin, and patients may benefit from several days of pre-treatment with topical corticosteroids. "In these situations, patients can experience a great deal of burning and stinging, whereas that is virtually eliminated with pre-treatment," Dr. Fleischer notes.
And although tacrolimus and pimecrolimus have exactly the same mechanism of action, the two drugs are not equal in efficacy. Compared to pimecrolimus, tacrolimus binds at the target receptor three times the binding affinity and more quickly permeates the skin. However, Dr. Fleischer is quick to point out that a choice of drugs is necessary to satisfy the needs of individual patients, who, for example, may prefer to use a cream (pimecrolimus) over an ointment (tacrolimus).
"Given the choice," he says, "most patients will choose a more effective ointment over a less effective cream. But, it is important to have a choice of agents. No individual patient responds ideally to a single drug."
Because of the number of chronic skin conditions that respond to anti-inflammatory treatment, the implications regarding use of calcineurin inhibitors are broad. Diseases that require long-term treatment including psoriasis, seborrheic dermatitis, alopecia areata, lichen sclerosis, lichen planus, vitiligo and a host of other conditions have been shown to be responsive to tacrolimus and pimecrolimus therapy.
Dr. Fleischer points out that off-label prescribing in dermatology is common, and constitutes the majority of his use of tacrolimus and pimecrolimus.






