- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Article
Steroid-free atopic dermatitis topical completes phase 4 trial
Author(s):
Crisaborole ointment, 2%, (Eucrisa, Pfizer) appears to be safe and effective in children aged three months to less than 24 months who have mild-to-moderate atopic dermatitis, according to results of a phase 4 trial released yesterday.
(©Skylines/Shutterstock.com)
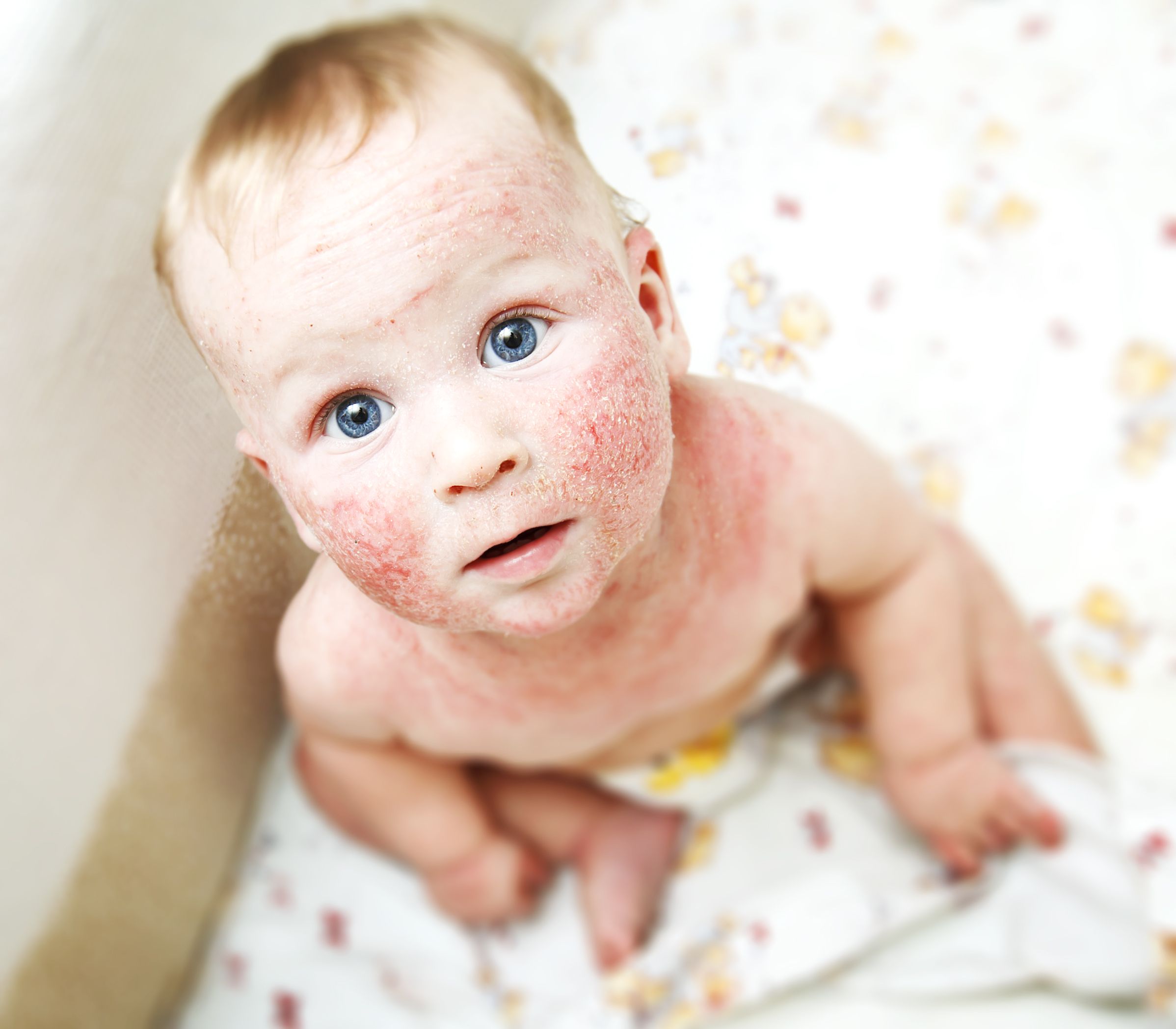
Crisaborole ointment, 2%, (Eucrisa, Pfizer) appears to be safe and effective in children aged three months to less than 24 months who have mild-to-moderate atopic dermatitis, according to results of a phase 4 trial released yesterday.
Crisaborole ointment, 2%, is a steroid-free topical phosphodiesterase inhibitor. The CrisADe CARE 1 trial examined the number of patients with treatment-emergent adverse events and severe adverse events, and the number of patients with clinically significant changes from baseline in height, weight, vital signs, electrocardiogram, and clinical laboratory parameters, according to the press release.
“This study reinforces our commitment to young AD patients worldwide who need more options,” said Michael Corbo, Chief Development Officer, Inflammation & Immunology, Pfizer Global Product Development. “These results add to the growing body of evidence that underscores the strength of data for crisaborole as a steroid-free, topical treatment option for people with mild to moderate atopic dermatitis.”
The full study details will be submitted for presentation at a scientific meeting in the future.
Crisaborole ointment, 2%, is currently approved in the U.S. and Canada (Eucrisa, Pfizer) and Israel and Australia (Staquis, Pfizer) to treat mild to moderate atopic dermatitis in pediatric patients two years and older.






