- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Article
Psoriasis cream safe, effective suggests EU phase 3 results
Author(s):
Results from an EU phase 3 clinical trial of a combination of betamethasone dipropionate and calcipotriene cream showed improved patient convenience, quality of life and efficacy compared to a betamethasone dipropionate and calcipotriene gel for patients with plaque psoriasis.
Results from an EU phase 3 clinical trial of a combination of betamethasone dipropionate and calcipotriene cream showed improved patient convenience, quality of life and efficacy compared to a betamethasone dipropionate and calcipotriene gel for patients with plaque psoriasis. (Ольга Тернавская - stock.adobe.com)
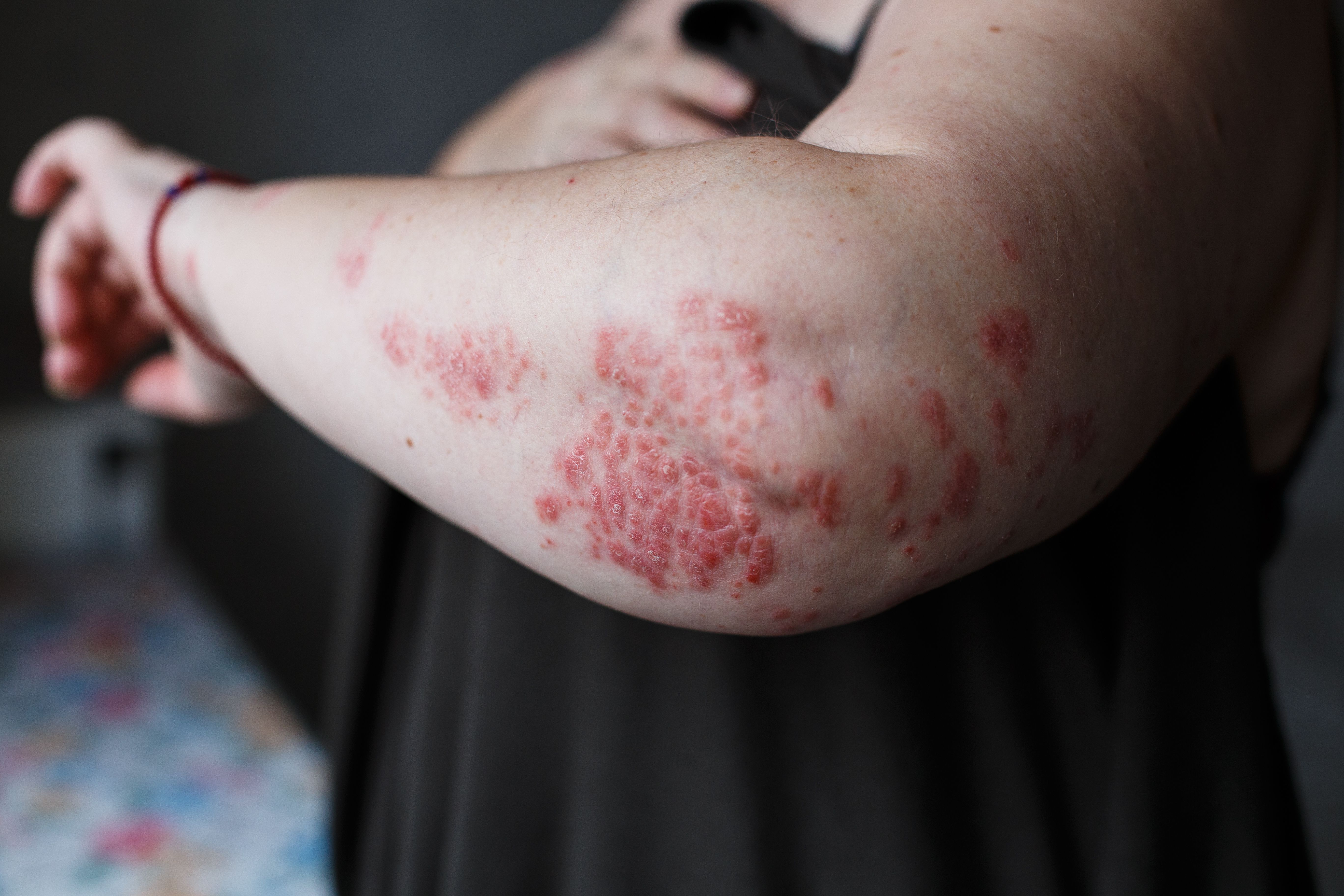
An investigational anti-inflammatory cream from MC2 Therapeutics may offer an improved treatment option for psoriasis patients, according to recently released EU phase 3 trial results.
RELATED: Bimekizumab offers hope for skin clearance in psoriasis
Investigators compared the efficacy and safety of a combination of betamethasone dipropionate and calcipotriene cream (Wynzora, MC2 Therapeutics) to an approved betamethasone dipropionate and calcipotriene gel (Taclonex, Leo Pharma).
The topical, fixed dose combination of betamethasone dipropionate and calcipotriene cream (0.005%/0.064% w/w) utilizes MC2 Therapeutics’ patented PAD Technology.
PAD Technology allows calcipotriene and betamethasone dipropionate to remain stable in an aqueous form that quickly absorbs into the skin without feeling greasy, according to the company.
During the trial, researchers found the cream met all primary endpoints, including establishing improved quality of life, convenience and efficacy compared to the gel.
“Wynzora Cream takes the treatment experience in daily routines to a new level. Our PAD Technology has enabled an aqueous cream formulation that is designed for high convenience in daily routines illustrated by the positive patient reported treatment convenience and quality of life outcomes in our phase 3 trials. This is a key component of treatment in real life settings,” says Jesper J. Lange, CEO, MC2 Therapeutics.
The study examined 490 plaque psoriasis patients across 32 clinical centers in Poland, Germany and Czech Republic, where researchers had participants apply the cream once daily for eight weeks.
READ MORE: Comorbidities more prevalent in pediatric psoriasis patients
Results showed the betamethasone dipropionate and calcipotriene cream displayed greater efficacy in comparison to the gel with a Physician Global Assessment (PGA) treatment success of 52% at week 8 and while also exhibiting a 68% reduction from the modified Psoriasis Area and Severity Index (mPASI) baseline at week 4. The topical also showed a favorable safety profile in the phase 3 study.
Additionally, MC2 Therapeutics’ new cream revealed better patient reported outcomes compared to the topical gel with p<0.001 Patient Reported Treatment Convenience (PTCS) at week 4 and p<0.0001 at week 8, as well as an alteration from the Dermatology Life Quality Index (DLQI) baseline with p<0.05 at week 4 and 8.
“We continue to be impressed by the performance of Wynzora Cream in clinical trials. With a PGA treatment success of 52% and 68% reduction in mPASI and a very favorable safety profile, Wynzora Cream is delivering on its promise to provide high comfort to physicians and patients in treating plaque psoriasis,” states Lange.
Complete data results from the study will be presented at clinical conferences in the upcoming year. MC2 Therapeutics also plans to apply for marketing authorization in the EU during the first quarter of 2020.
References:
MC2 Therapeutics Announces Positive Top-line Results from EU Phase 3 Head-to-Head trial Comparing Wynzora™ Cream to Daivobet® Gel in Patients with Psoriasis. mc2therapeutics.com. https://www.mc2therapeutics.com/news/?nid=5df9ddee44c43. Published December 18, 2019. Accessed December 20, 2019.





