- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Article
The evolving landscape of pediatric psoriasis
Author(s):
About one-third of patients with psoriasis are children or adolescents. Still, little research exists on treating this patient population. New disease management guidelines and emerging studies seek to fill the gap.
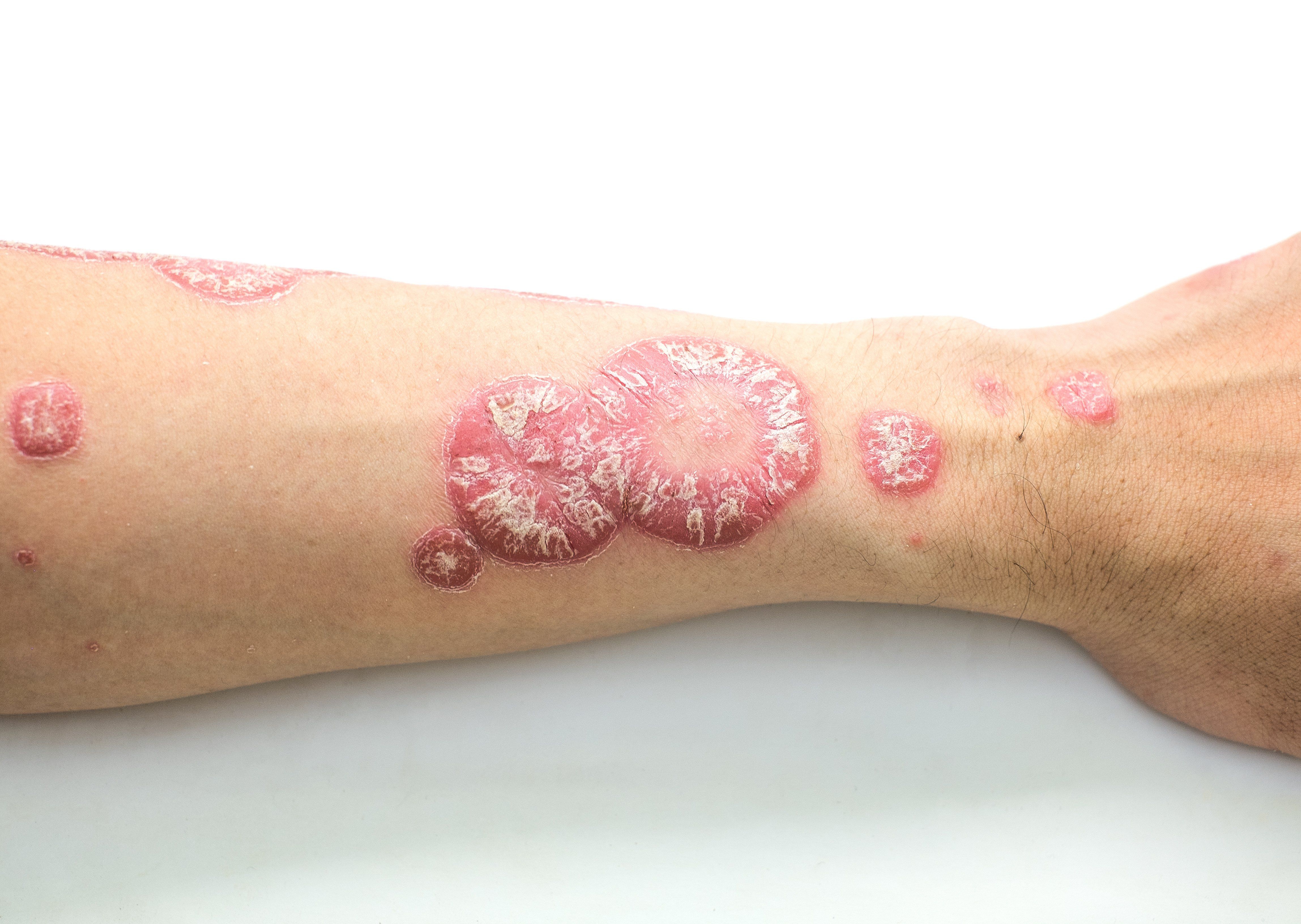
Research suggest possible differences in the pathogenesis of pediatric and adult psoriasis and in targets for therapy, says Dr. Paller. (baworn47 - stock.adobe.com)
Dr. Paller

Psoriasis has been estimated to affect up to 3% of the population. For the majority of patients, onset occurs before the age of 40, and up to one-third of cases begin in childhood. Related to these data about the epidemiology of psoriasis are unique questions about its treatment and other issues in the pediatric population.
According to Amy S. Paller, M.D., a need for more research aptly describes the current state of knowledge about multiple aspects of psoriasis in the pediatric population. However, there have been some recent positive developments, she says.
Dermatologists can look forward to the upcoming release of Joint American Academy of Dermatology-National Psoriasis Foundation (AAD-NPF) guidelines of care for the management of psoriasis in pediatric patients, which are expected to be published later in 2019.
Read about comorbidity screening guidelines here.
“It is exciting that we will be having a new set of management guidelines coming out in psoriasis and doubly exciting that for the first time we will have guidelines dedicated to the pediatric population,” says Dr. Paller, Walter J. Hamlin Professor and chair of dermatology, Northwestern University Feinberg School of Medicine, Chicago. “There are unique physiological and social considerations for pediatric patients with psoriasis relative to the adult population, and so it is important to have a separate set of guidelines for psoriasis management in children.”
Expert opinion based on the experience of the guideline working group members was important for developing the guideline’s treatment recommendations considering the limited evidence on treatment of pediatric plaque psoriasis.
“There is some mid-level evidence on the use of biologic agents for treating psoriasis in children, but high-quality trials providing information about the safety and efficacy of other treatments are more limited,” Dr. Paller says.
Only a few treatments have an FDA-approved indication for treatment of psoriasis in children. They include a topical combination corticosteroid-vitamin D3 analog (calcipotriene and betamethasone dipropionate 0.005%/0.064%, Taclonex, LEO Pharma), ustekinumab (Stelara, Janssen), and etanercept (Enbrel, Amgen), but the topical product and ustekinumab are only approved in adolescents aged 12 years and older.
The situation is changing slowly.
A recent phase 3 study compared methotrexate and adalimumab in pediatric patients and the results favored the biologic.1 It should be noted, however, that methotrexate was administered at a relatively dose (mean of ~0.15 mg/kg once weekly), Dr. Paller says.
There have been published studies investigating ustekinumab in adolescents, and studies of adalimumab (Humira, AbbVie) have been conducted outside of the United States. In addition, studies are ongoing to evaluate ixekizumab (Taltz, Eli Lilly) in adolescents, ustekinumab in children 6-11 years old, and guselkumab (Tremfya, Janssen) in adolescents.
“Hopefully this research will lead to approval of more biologics for the treatment of psoriasis in children. To date, we have been treating children with biologics off-label when it is possible to obtain authorization, but our approaches are based on what we know from studies in adults,” Dr. Paller says.
The expansion that is occurring in biologic therapies for psoriasis underscores the need for more basic science research on pediatric psoriasis. Potential age-related differences in immunopathology may have implications for treatment selection, Dr. Paller says.
In that regard, she notes that a small pilot study analyzing skin samples of plaque psoriasis using flow cytometry found that affected children had significantly higher levels of IL-22 expressing cells and significantly lower levels of IL-17 expressing cells than affected adults.2
“These findings suggest possible differences in the pathogenesis of pediatric and adult psoriasis and in targets for therapy,” Dr. Paller says.
There are also gaps in knowledge about medical comorbidities in pediatric patients with psoriasis that need to be addressed with more research.
It is clear that there is a disproportionate prevalence of obesity among adolescents with psoriasis compared with the general population, and even younger children with psoriasis seem more likely to be obese than their unaffected peers.
More on obesity, psoriasis link.
According to the results of at least one study, obesity in children with psoriasis preceded onset of the skin disease in more than 90% of the children.3
“The comorbidity of obesity with psoriasis begs the question of whether earlier intervention to control psoriasis will prevent the later development of cardiovascular and metabolic issues that are related to obesity. Studies addressing this issue are being conducted in adult populations, but it will be many years before we know the answer in children,” Dr. Paller says.
She adds that growth in patient registries, which provide a resource for longitudinal follow-up, is a positive development for helping to answer this and other questions.
“A longitudinal registry for children with psoriasis in the U.S. is an unmet need,” Dr. Paller says.
Disclosures:
Dr. Paller serves as consultants to and receive honoraria as speakers for companies that market products used for the treatment of psoriasis.
References:
1. Papp K, Thaçi D, Marcoux D, et al. Efficacy and safety of adalimumab every other week versus methotrexate once weekly in children and adolescents with severe chronic plaque psoriasis: a randomised, double-blind, phase 3 trial. Lancet. 2017;390(10089):40-49.
2. Cordoro KM, Hitraya-Low M, Taravati K, et al. Skin-infiltrating, interleukin-22-producing T cells differentiate pediatric psoriasis from adult psoriasis. J Am Acad Dermatol. 2017;77(3):417-424.
3. Becker L, Tom WL, Eshagh K, Benjamin LT, Paller AS. Excess adiposity preceding pediatric psoriasis. JAMA Dermatol. 2014;150(5):573-574.






